Here are some links to help you cram!
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Stoichiometry_and_Balancing_Reactions
http://www.chemcollective.org/activities/tutorials/stoich/reaction_stoi
http://www.chemteam.info/Stoichiometry/Stoichiometry.html
http://www.chem4kids.com/files/react_stoichio.html
http://www.chemtutor.com/mols.htm
Monday, December 14, 2015
Post- Lab
Here are the calculations that I did after lab in order to figure out if we were given Fe2+ or Fe3+, we had Fe3+. This practice was very helpful and helped me physically see the process of how the data is collected and how we get the answers we do.
Monday, December 7, 2015
Stoichiometry: The Basics
So far we have learned that stoichiometry is taking a balanced chemical equation and finding the quantities of the reactants and or products. If you can identify what you are given and what you are looking for, then the problems should be pretty simple.
Here is a link that helps go into more depth about stoichiometry:
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/a/stoichiometry
Here is a link that helps go into more depth about stoichiometry:
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/a/stoichiometry
Thursday, December 3, 2015
End of unit
As the unit comes to an end with the unit test today, I feel pretty good about most of the subjects. There are a couple things that I may need to study harder for the final, but I feel more confident in ending this unit than I did last unit!
Wednesday, December 2, 2015
Helpful Links to Use When Cramming!
These are some of the helpful websites i have been visiting in order to study for tomorrows test, i hope they help!
Balancing equations:
http://www.webqc.org/balance.php
http://education.jlab.org/elementbalancing/
Double replacement:
http://www.chemteam.info/Equations/DoubleReplacement.html
Redox reactions:
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
acid base reactions:
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/acidbase.html
Synthesis reactions:
http://chemistry.about.com/od/chemicalreactions/a/Synthesis-Reactions.htm
Balancing equations:
http://www.webqc.org/balance.php
http://education.jlab.org/elementbalancing/
Double replacement:
http://www.chemteam.info/Equations/DoubleReplacement.html
Redox reactions:
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
acid base reactions:
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/acidbase.html
Synthesis reactions:
http://chemistry.about.com/od/chemicalreactions/a/Synthesis-Reactions.htm
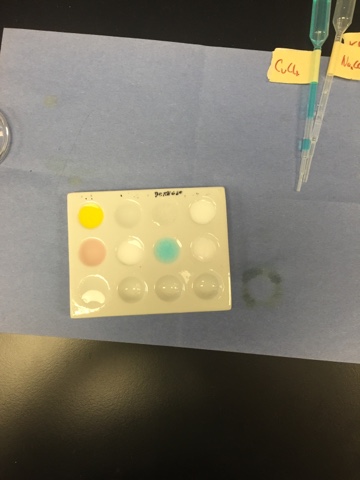
Redox Lab
In this lab, we again combined two substances, but this time one was a solid metal and the other was an aqueous solution. This lab helped me to visually see what happens when a reaction occurs showing a color change, a formation of a gas, or bubbling. Some reacted, but almost half didn't. All and all, I enjoyed this lab!
Tuesday, November 24, 2015
Acid Base reactions
https://www.youtube.com/watch?v=ANi709MYnWg
Acid Base reactions occur when water is the riving force in the reaction. The products include "salts" and water. There are rules for strong acids strong bases as well as weak acids and bases. The video link attached is a video from crash course chemistry on the subject and helps explain further with helpful examples. I hope this helps!
Acid Base reactions occur when water is the riving force in the reaction. The products include "salts" and water. There are rules for strong acids strong bases as well as weak acids and bases. The video link attached is a video from crash course chemistry on the subject and helps explain further with helpful examples. I hope this helps!
Monday, November 23, 2015
Chemical Reactions Lab
In lab on Friday, we used two aqueous substances to mix together to show the formation of a precipitation. I enjoyed this lab because it showed me first hand how some solutions can form solids and some may not! Either way it was an interesting lab!
Thursday, November 19, 2015
Balancing Chemical Equations
http://www.wikihow.com/images/2/2f/Balance-Chemical-Equations-Step-6-preview.jpg
In class yesterday we learned about how to balance chemical equations, which i remember from physical science so it wasn't too hard to pick back up. The main thing to remember is too make sure that you have the same amount of elements on each side since in chemical reactions, elements don't just appear out of no where, although they can form new compounds. I know this unit will get harder as we go along, but so far its not too bad!
Sunday, November 15, 2015
Helpful Links to Cram With
These are the links ive been using to study the night before the big test, i hope they help!
Empirical Formula:
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://www.chemteam.info/Mole/EmpiricalFormula.html
Hydrates:
http://www.chemteam.info/Mole/Determine-formula-of-hydrate.html
http://study.com/academy/lesson/hydrates-determining-the-chemical-formula-from-empirical-data.html
Molar Mass:
Empirical Formula:
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://www.chemteam.info/Mole/EmpiricalFormula.html
Hydrates:
http://www.chemteam.info/Mole/Determine-formula-of-hydrate.html
http://study.com/academy/lesson/hydrates-determining-the-chemical-formula-from-empirical-data.html
Molar Mass:
Friday, November 13, 2015
Study Tools
Here are some pictures and videos that i have been using to study for the big test on monday. I hope they help!
Hydrate Video

http://nonsibihighschool.org/intbasch3_files/intbasch3pracex3-4.png
Empirical formula
 \
\
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/emp1c.gif
Hydrate Video
http://nonsibihighschool.org/intbasch3_files/intbasch3pracex3-4.png
Empirical formula
 \
\https://www.chem.tamu.edu/class/majors/tutorialnotefiles/emp1c.gif
Thursday, November 5, 2015
The Mole

https://a-woods.wikispaces.com/file/view/molar_roadmap.gif/251028280/800x515/molar_roadmap.gif
Yesterday and today in class we learned about the unit of measurement, the mole. Molar quantities are measured in this. Dimensional analysis, which is what we learned last unit, is an extremely useful skill when converting chemical compositions in this unit. So far i have understood most if not all of what we have learned so far this unit, but ill still be studying!
Wednesday, November 4, 2015
Pre test frightening fears
As many of my peers have said, the pretest for this unit made absolutely no sense. I don't remember learning about any of what was on the test. I know that I need to make sure I'm studying and asking questions to get through this unit!
Also, after our first lecture today, some if not most of what was on the test made more sense from our lesson about the mole today. I will have a later post explain what I've learned about the mole!
Wednesday, October 28, 2015
More links for the test
Here are some more links to help you on the test that i found today! I hope they help!
Density conversions:
https://www.youtube.com/watch?v=8ePH-wsaalo
http://chemistry.alanearhart.org/Old/Tutorials/DimAnal/dimanal-part7.html
Density conversions:
https://www.youtube.com/watch?v=8ePH-wsaalo
http://chemistry.alanearhart.org/Old/Tutorials/DimAnal/dimanal-part7.html
Tuesday, October 27, 2015
Helpful links for the big day
Here are some helpful videos and links that have helped me review and study fro the test on Thursday. I hope these help!
Dimensional analysis:
https://www.youtube.com/watch?v=aZ3J60GYo6U
 https://sophialearning.s3.amazonaws.com/packet_logos/53911/grid/DimensionalAnalysisImage2.jpg?1348236631
https://sophialearning.s3.amazonaws.com/packet_logos/53911/grid/DimensionalAnalysisImage2.jpg?1348236631
http://www.chem.tamu.edu/class/fyp/mathrev/mr-da.html
Matter and measurement:
http://wps.prenhall.com/esm_hillpetrucci_genchem_4/16/4213/1078774.cw/index.html
Dimensional analysis:
https://www.youtube.com/watch?v=aZ3J60GYo6U
 https://sophialearning.s3.amazonaws.com/packet_logos/53911/grid/DimensionalAnalysisImage2.jpg?1348236631
https://sophialearning.s3.amazonaws.com/packet_logos/53911/grid/DimensionalAnalysisImage2.jpg?1348236631http://www.chem.tamu.edu/class/fyp/mathrev/mr-da.html
Matter and measurement:
http://wps.prenhall.com/esm_hillpetrucci_genchem_4/16/4213/1078774.cw/index.html
Friday, October 23, 2015
Scientific Notation
This photo, as well as quiz, helped me review and study when learning scientific notation. Scientific notation is important to know and not something to just pass over since we will be using it for the rest of the year. I'm not worried too much about how to write scientific notation though considering I remember it from physical science. I hope this helps!
Wednesday, October 7, 2015
Aspirin Lab Day 1
Today, once my lab partner and I passed the prelab question, we conducted the first 4 steps in the procedure for making aspirin. We used 5mg of salicilic acid, and carefully put 7mL of acetic anhydride into that flask, then put the flash into a hot water bath for 15 minutes. The substance turned dark green, then brown once we added the 8 drops of sulfuric acid. The crystals are normally supposed to be white, but hopefully our crystals will turn out well tomorrow!
Tuesday, September 29, 2015
Helpful links
As I'm studying, i have been placing links, pictures, and videos that may help others for this upcoming Unit test here:
http://www.chem4kids.com/files/atom_structure.html - here is an easy outline of the atom's structure
 https://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm - table and site that will go more in depth with atomic structure
https://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm - table and site that will go more in depth with atomic structure

http://www.chem4kids.com/files/atom_structure.html - here is an easy outline of the atom's structure
 https://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm - table and site that will go more in depth with atomic structure
https://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm - table and site that will go more in depth with atomic structure
http://vignette2.wikia.nocookie.net/schools/images/0/0b/Chart.JPG/revision/latest?cb=20060611033958 - here is a picture that will hopefully help outline most of the aspects to radioactive decay
here is a useful link and video that will help outline and explain the different types of decay
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/modes.php another link to help with radioactive decay
https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties -khan academy is a excellent site to explore the atom in different ways and videos
https://en.wikipedia.org/wiki/Plum_pudding_model i had trouble with the difference between Johnson's model and the rest, so i hope this link is helpful
I hope these links, pictures, and videos have helped you study just like they helped me.
Wednesday, September 23, 2015
Post- Weekly quiz #1
We took the first quiz of the atomic structure and radioactivity today and i felt like i knew the majority of the content. I realized afterwords that i did not calculate the average atomic mass correctly, but i now reviewed the equation and have corrected my mistake for the unit test. Otherwise, I have understood this unit and haven't had very many problems so far.

http://i.ytimg.com/vi/HIToHoZGRSE/hqdefault.jpg
http://i.ytimg.com/vi/HIToHoZGRSE/hqdefault.jpg
Tuesday, September 22, 2015
Beanium Lab
Today we did a lab replacing isotopes and atoms with beans. This lab helped me understand the total average mass equation that we learned yesterday because I was physically working out the problem and visualizing the beans as the isotopes. I also learned that the total average mass of the four isotopes should be closest to the more abundant isotopes mass.
Monday, September 21, 2015
Mass of Subatomic Particles
Today the notes we learned about were more based on the equation of determining either the percent abundance or average mass, which has the equation: [(mass)(%)]+[(mass)(%)]=total average mass. This equation is easy to understand, but i will need to do some practice some problems to be able to easily use the equation when need be.
Sunday, September 20, 2015
First in-Class Notes
After our first in-class lecture, i am excited to begin the math part of Chemistry. I am also excited to learn about an atom in more detail to fully understand its properties and how the discovery of all of those aspects came along over time. By the end of this unit, i hope to fully understand all of the concepts through studying and reviewing what I have learned.
Pre Test
I took the pre-test last week which made me realize that i don't have an abundance of prior knowledge for this unit, but i do remember some terms such as half-life or gamma radiation from my previous science classes. I am excited to learn more throughout this unit and for this upcoming project with being able to study the stars and aspects of space.
Monday, September 14, 2015
Reflection on Nomenclature
I learned in the Nomenclature Unit that there are three different types of ways to name ionic compounds and covalent compounds. When naming acids, I remember "No O go hydro", so if there is oxygen in the compound, there wont be a hydro-suffix.
Thursday, August 20, 2015
Introduction Page
My name is Celine Jennings. My future goals are to attend Washington University in St. Louis, and to become a Surgeon. In my free time I like to work as a lifeguard and spend time with my friends.
Subscribe to:
Comments (Atom)